Document Type : Original Research
Authors
1 Dept. of Anatomy, Molecular and Cell Biology Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
2 Dept. of Anatomy, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
3 Dept. of microbiology, Islamic Azad University, Qaemshar, Iran
Keywords
Introduction
Apoptosis is a Programmed Cell Death (PCD), which arises from a series of specific biochemical events. This process is a fundamental physiol-ogical phenomenon, which is involved in controlling the balance between proliferation and differentiation during development, and in the optimization of cell/tissue functions throughout adulthood (1).On the other hand, inappropriate apoptosis is a factor in many human conditions including neurodegenerative diseases, ischemic damage, autoimmune disorders and many types of cancer (2, 3). Apoptosis can be triggered by numerous types of cellular damage and derangement, via the intrinsic apoptotic pathway (4).Meanwhile, apoptosis is an important mediator of secondary damage after traumatic injury, which is triggered by varieties of mechanisms, including free radical damage, cytokines, and inflammatory injury (5). In the recent years, much attention has been focused on apoptosis because it appears to be susceptible to therapeutic interventions with anti-apoptotic agents. Each treatment, which interrupts the apoptosis processes, could improve the pathological condition. In this regard, in the previous decades, a rapidly growing number of natural polyphenol compounds with anti-apoptotic effects have been described. One of the main sources of these molecules is olive oil. Olive oil is a rich source of polyphenolic components, such as its main component oleuropein (3, 4 dihydroxyphenylel-enolic acid), which have many beneficial health effects in humans (6, 7). In addition, olive oil phenols have been shown to be involved in some of the protective effects against ischemia and neurodegenerative diseases such as Parkinson’s (8) and Alzheimer’s (9) disease. There is accumulating evidence that have attributed the beneficial effects of oleuropein and its derivatives to a variety of biological activities, including free radical scavenging/antioxidant actions, anti-inflammatory effects, and anti-carcinogenic properties (10, 11). On the other hand, some experimental studies have documented that oleuropein and its derivatives have anti-apoptotic effects against intestinal ischemia/reperfusion injury (12), 6-hydroxydopamine-induced PC12 cell apoptosis (13), and doxorubicin-induced cardiomyopathy (14). Recently, we documented that oleuropein have protective effects against acute deltamethrin-induced neurotoxicity, which was partly due to alternation of apoptosis regulating proteins (15). In the present study, we exclusively investigated the potential anti-apoptotic effect of oleuropein in dexamethasone-induced thymocyte apoptosis, as a standard model of apoptosis induction, with immunohistochemical and electron microscopic assessment.
Materials and Methods
Animals
Four-week-old Balb/c mice (10–12g) were used (laboratory animal research center, Sari, Iran) in this study. They were kept under standard conditions and were fed a standard mice chow and drinking water ad libitum throughout the study period.
Experimental groups
The mice were randomly allocated to four groups, each with 5 mice: (i) Dexamethasone-treated group (Dex), which received a 20-mg/kg single dose of dexamethasone (Sigma)(16);(ii) Oleuropein-treated group (Ole), which received 20-mg/kg of oleuropein (Sigma) for 7 days (14); (iii) Dex plus Ole-treated group, which received 20-mg/kg of oleuropein for 7 days and then a 20-mg/kg single dose of dexamethasone on the seventh day; (iv) Vehicle group, which received saline.
Histological assessment
Sixteen hours after dexamethasone injection (17), the entire thymus was removed under ether anesthesia through a longitudinal midline incision and median split of sternum from the manubrium to the level of 2nd intercostal space. Five-micrometer serial transverse sections were prepared from the paraffin-embedded blocks with the use of a microtome. For histopathological assessment, 10 sections of each block were randomly selected, deparaffinized with xylene, stained with Hematoxylin Eosin (H&E), and studied using light microscopy (DME; Leica Microsystems Inc., Buffalo, NY, USA). An average of 5 fields in each tissue section was evaluated. All the histological studies were performed in a blinded fashion.
Immunohistochemistry assessment
For immunohistochemistry, sections were incubated in goat serum (in order to block nonspecific site), and anti-Bax rabbit polyclonal antibody (1:50 in PBS, vol. /vol., Abcam), or anti-Bcl-2 rabbit polyclonal antibody (1:100 in PBS, vol. /vol., Abcam) overnight at 4°C. Sections were washed with PBS and then incubated with secondary antibody conjugated with horseradish peroxidase (goat anti-rabbit IgG peroxidase, Abcam) for 2 hours and visualized with diaminobenzidine tetrahydrochloride for 5 minutes. Afterwards, they were dehydrated and mounted. For negative controls, primary antibodies were omitted. For quantitative analysis, immunohistochemical photographs (n=5 photos from each sample collected from all mice in each experimental group) were assessed by densitometry using MacBiophotonics Image J 1.41a software on an ASUS personal computer.
Electron microscopic assessment
Transmission Electron Microscopic (TEM) analysis has been considered the ‘golden standard’ in cell death research. In brief, for transmission electron microscopy study, small sections (approximately 1 mm3) of freshly excised thymus tissues were fixed in 2.5% glutaraldehyde, followed by 1% osmium tetroxide and then embedded in epon araldite. The 50-nm slices were analyzed and photographed with a Zeiss electron microscope after staining with uranyl acetate and lead citrate.
Statistical analysis
Statistical analysis was carried out using the SPSS software (Version 15, Chicago, IL, USA). Results were presented as mean values (±SDM). The K-S test was used in order to evaluate the normality of the data. Also, the Tukey's multiple comparison tests and the analysis of the variance were used in order to compare each of the two groups and compare the data among the groups, respectively. A value of p<0.05 was considered significant.
Results
Histological changes of thymocytes
To observe the morphological characteristics of thymocytes in thymus of all experimental groups, hematoxylin-eosin staining was used in the present study. Histopathological study with hematoxylin-eosin staining has shown some degenerative changes in the vast majority of cortical thymocytes (shrinkage of individual thymocytes and condensation of nuclear chromatin) (Fig. 1A) in the thymus of Dex- treated mice. However, little signs of the degeneration were seen in Dex plus Ole-treated group (Fig. 1B) and in the Ole treated group or in the vehicle group.


Fig 1. Hematoxylin Eosin Staining of Paraffin Sections from the Thymus of Dex (A) and Dex+Ole (B) treated-mice Many thymocytes showed characteristics of apoptosis with pyknosis of nuclei and shrinkage of cytoplasm in Dex group (arrow), 1000×. Little signs of degeneration were seen in Dex + Ole group (arrow), 1000×.
Ultrastructural changes of thymocytes
Figure 2 is a transmission electron micrograph of the thymus tissue. Figure 2A shows some normal thymocytes, which is closely packed, has large nuclei and scant cytoplasm. Figure 2B is an apoptotic thymocytes in an early phase of apoptosis with condensed and peripheralized chromatin. The cytoplasm is beginning to condense and the cell outlines are irregular. It was observed that a lot of the thymocytes of Dex group displayed specific morphological changes, including reduction of cell volume, condensation of chromatin, and flocculation of the cytoplasm. However, little signs of apoptosis were seen in the Dex plus Ole group. These morphological modifications were not observed in Ole and vehicle group.
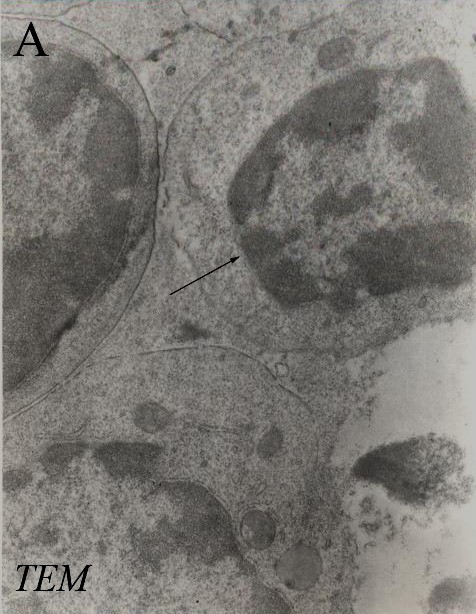

Fig 2. Transmission Electron Micrographs from the Thymus of Dex+Ole (A) and Dex (B) Treated-Mice. Figure A shows some normal thymocytes with large nuclei and scant cytoplasm (arrow), 3000×. Figure B shows a thymocyte with characteristics of apoptosis (condensed and peripheralized chromatin) (arrow), 3000×.
Immunostaining changes for Bax and Bcl-2 of thymocytes
Figure 3 shows the immunohistochemical staining of Bax. Thymocytes from Dex treated-mice exhibited strong positive staining for Bax(10.94±3.35) (Fig. 3A); whereas, oleuropein treatment in Dex plus Ole treated-mice reduced the degree of positive staining for Bax (2.64±1.26) (Fig. 3B). Sections of Ole (0.65±0.30) and vehicle (0.67±0.29) treated-mice showed weak positive immunoreactions for Bax..


Fig 3. Light Photomicrographs Show Immunohistochemical Expression of Bax in Dex (A) and Dex+Ole (B) groups (arrow), 1000× the positive staining of Bax is presented by a brown color of cytoplasm.

Fig 4. Light Photomicrographs Show Immunohistochemical Expression of Bcl-2 in Dex (A) and Dex+Ole (B) groups (arrow), 1000× The positive staining of Bcl-2 is presented by the brown color of the cytoplasm

Fig 5. Densitometry Analysis of Immunohistochemical Photomicrographs for Bax

Fig 6. Densitometry Analysis of Immunohistochemical Photomicrographs for Bcl-2
Data are expressed as a percentage of total tissue area. *P<0.001 versus Dex group; #P>0.05 versus Ole group; **P<0.01 versus Vehicle group. Bars indicate the standard deviations of the mean (SDM).
Figure 4 shows the immunohistochemical staining of Bcl-2. The expression of Bcl-2 was strong in thymocytes from Ole (14.94±1.54) and vehicle (18.93±3.54) treated-mice, while, it was weak in the Dex treated-mice (2.94±0.42) (Fig. 4A) compared to the up-regulation in the Dex plus Ole treated-mice (12.24±1.84) (Fig. 4B).
Quantitative analysis
The histograms of the quantitative analysis of Bax and Bcl-2 staining in the experimental groups are shown in Figures 5 and 6, respectively.
Discussion
Apoptosis is a key molecular mechanism of some degenerative diseases and toxicities, and is regulated by the Bcl-2 family proteins (2). Among these proteins, Bcl-2 and Bax play anti-apoptotic and pro-apoptotic roles, respectively (18). The ratio of Bax to Bcl-2 determines the cell fate; excess Bcl-2 leads to survival of cells, while Bax induces apoptosis (19, 20). Results of our immunohistochemical assessment showed that treatment with dexamethasone increased positive staining for Bax, while it exhibited a decreased positive staining for Bcl-2 in thymocytes of Dex group. These represent a potentially avoidable event by pharmacological interventions. To date, the majority of epidemiological studies involving olive oil are linked to a decreased incidence of certain types of degenerative diseases (21-23). On the other hand, animal and human studies demonstrated that olive oil phenolic compounds are highly bioavailable; the first requirement for a dietary compound to have a potential protective effect is that it enters the blood circulation. In this regard, a recent study showed that after a single ingestion of olive oil phenolic compounds, these were absorbed, metabolized and distributed through the blood stream to practically all parts of the body of the rat (24). In vitro studies have suggested that anti-apoptotic properties of oleuropein and its derivatives, is a pivotal potential protective mechanism against degenerative diseases (25). Results of our immunohistochemical assessment showed that treatment with oleuropein reduced positive staining for Bax; while on the contrary, it increased positive staining for Bcl-2 in the Dex plus Ole-treated group. Conversely, oleuropein inhibited the expression of proapoptotic protein Bax and induced that of the antiapoptotic protein Bcl- 2, thereby provided molecular evidence for the protective activity of oleuropein against apoptosis. In this regard, González-Correa et al. documented that lactate dehydrogenase efflux, as a marker of cell death, was inhibited, in a concentration-dependent manner after 7 days of oral treatment with hydroxytyrosol, in rat brain slices subjected to hypoxia-reoxygenation (26). An in vitro study has shown that the olive oil phenolic extract and one of its constituents, gallic acid, exert anti-apoptotic effect against H2O2-induced apoptotic cell death in Hela cells with reduction of time-dependent caspase 9 activities (27). Also, another study documented that incubation of PC12 cells with oleuropein could decrease cell damage and reduce biochemical markers of apoptotic cell death including activated caspase 3 and DNA fragmentation in 6-hydroxydopamine-induced PC12 cell apoptosis (13). An in vivo study and molecular examinations demonstrated that oleuropein aglycone modulated an apoptosis pathway, as shown by tunnel staining, in a murine model of intestinal ischemia/reperfusion injury (12). A recent study has shown that oleuropein prevents doxorubicin-induced cardiomyopathy through modulation of kinases such as Akt (28), a serine/threonine-specific protein kinase that plays a key role in apoptosis and cell proliferation (29).
There is some evidence that production of free radicals plays a critical role in dexamethasone-induced thymocytes apoptosis so that exogenous treatment with antioxidants or metal chelators protects these cells against apoptosis (30, 31). Free radicals are highly reactive molecules, defined as any chemical compound that has one or more unpaired electrons, which can have damaging effects directly on the cell, particularly on DNA, proteins, and lipids (32). These molecules have been implicated as a potential contributor to the pathogenesis of degenerative diseases. On the other hand, some studies documented that dexamethasone causes a down-regulation of several antioxidant defense enzymes (33). Oleuropein is an ortho-diphenol, with two adjacent hydroxyl groups to the ring structure (34). Antioxidant properties of ortho-diphenols are related to hydrogen-donation by forming an intramolecular hydrogen bond between the free hydrogens of their hydroxyl group and their phenoxyl radicals (35). Furthermore, oleuropein prevents free radical formation through its ability to chelate metal ions such as Cu and Fe, which catalyze free radical generation reactions (36). Meanwhile, total plasma antioxidant activity has also been reported to increase in humans, after the ingestion of olive oil phenolic compounds (37, 38).
Conclusion
In the present study, it is clear that oleuropein pre-exposure provided protection against dexamethasone-induced apoptosis approved by electron microscopic as a golden standard, and immunohistochemical criteria. In conclusion, since apoptosis is a possible mechanism involved in cell death shown in several degenerative diseases, oleuropein might be a potent therapeutic agent in some of these conditions.
Acknowledgements
This work was supported by Molecular and Cell Biology Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences.
Conflict of Interests: The authors declare that there was no conflict of interest.
| Article View | 1,131 |
| PDF Download | 954 |