Introduction
Proprotein convertases (PCs) are serine proteases that convert various growth factors, cell surface glycoproteins, receptors, and metalloproteinases into active forms (1). PCSK9 is an enzyme and the ninth member of proprotein convertases that activates other proteins and also plays a vital role in regulating low-density lipoprotein cholesterol (LDL-C) levels because of its ability to adjust the hepatic expression of LDL-R (2). PCSK9 is produced in many organs, including kidneys, intestine, endocrine pancreas, and brain, but most significantly in the liver (3). Recent studies have revealed its presence in cerebrospinal fluid (CSF) and in the atherosclerosis plaque (4).
The inhibition of PCSK9 may be a promising treatment option to reduce LDL-C levels, though the cost of PCSK9 inhibitors may make their wide use difficult (5) (Figure 1).
In this study, we demonstrate that inhibition of PCSK9 might enhance the anti-cancer effects of immune checkpoint inhibitors (ICIs) (6). Therefore, a better understanding of PCSK9's role in the cancerous pathways is extremely important (7). Since this marker has a significant role in different parts of body, it has been used as a pharmacological target in the past few years (8-10).
Immune checkpoints are potent antioxidant regulators that control cellular tolerance and prevent tumors. Immune checkpoints assist the immune system in response to infections and cancer, which may protect tissues from damage (11-13). The concept that the immune system might restrict tumor development and cancer, dates back to 1893, when William Cooley used live bacteria as an immunological stimulant to treat cancer patients (2). This natural biological cancer-prevention mechanism has already been identified and has been activated by the essential immune inspection chemicals in cytotoxic T cells (14). The CTLA-4, PD-1, and PD-L1 are the most widely studied inhibitory pathways, and by blocking them, we can activate the immune system to attack tumors (15-17). Ipilimumab [CTLA-4 monoclonal antibody (18)] was approved by the Food and Drug Administration (FDA) as the first ICI in 2011 (19). Checkpoint therapy for cancer includes strategies that enhance the immune system response against tumor cells by targeting these regulatory pathways (20).
On the other hand, we have ICIs that have emerged as one of the most promising types of immunotherapies on the horizon in recent years. ICIs are the latest breakthrough in oncology, providing a new treatment model for advanced solid tumors (21). This shifted the companies’ focus away from developing cancer-fighting therapies toward screening inhibitors, which aim to kill tumor cells by removing obstructive signals that block anti-tumor T cell responses (22). In summary, we believe blocking PCSK9 would be a promising approach for improving cancer immune checkpoint therapy (23).
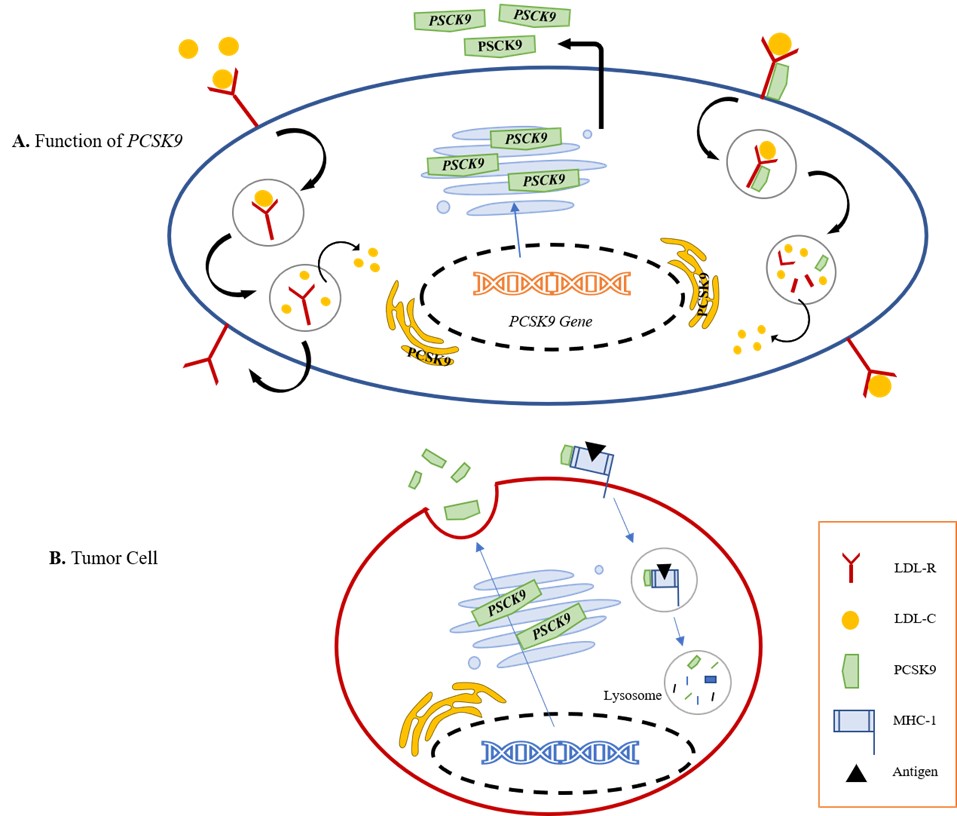
Fig. 1.A. Role of PCSK9 in increasing cholesterol availability for cancer cells (24). B. In tumor cells, upon binding to MHC I, PCSK9 mediates degradation via the endosomal/lysosomal pathway, preventing its recycling to the surface
Methods
We searched PubMed, NCBI, Scopus, and Google Scholar for the published articles without limitations on publication dates. The first search used the following terms: “PCSK9”, “Cancer”, “Immune Checkpoint” and “Cancer Prognosis” in the title and/or abstract. Our search initially revealed 600 records in used databases managed by the EndNote X8 software and we chose around 200 articles that were proper for our research. A total of 39 duplicate references were removed. The full texts of the remaining 161 articles were carefully reviewed and 76 of them were included in our research.
1. PCSK9 in Carcinoma
The discovery that PCSK9 interacts with LDL-R marked a significant advancement because it allowed the development of effective therapeutic strategies for cancer and other diseases (25). This section discusses PCSK9's potential as a biomarker for specific types of cancers.
1.1. PCSK9 and Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the fifth malignant neoplasm and the third most prominent cause of cancer death (26). The link between abnormal blood lipid levels and HCC has been established in clinical studies by He et al., (1, 27); They discovered that PCSK9 reduces HCC cell growth, cell cycle, and apoptosis in HepG2 cell line (a cell culture created from a single cell and contains cells with a consistent genetic makeup) by interacting with glutathione S-transferase p1 (GSTP1) and the c-Jun N-terminal kinase (JNK) signaling pathway (Up-regulated way) (28, 29). Still, the cell cycle study didn't find any G2/M phase arrest when PCSK9 was overexpressed or down-regulated (29). This means that PCSK9 doesn't have a big effect on how HCC cells divide (30). However, it has an impact on apoptosis with inhibitory effect (31). PCSK9 hinders the use of LDL and triglycerides by destroying LDLR (32). Fatty acid synthase (FASN) is expressed more often when PCSK9 is present (26, 30). It plays a crucial role in the synthesis of fatty acids from the beginning (29, 33). It also has a significant impact on the apoptosis of a variety of tumor types (34). Nevertheless, PCSK9 expression is unaffected by FASN blocking, which may lessen the anti-apoptotic impact (26). These results imply that FASN is downstream of PCSK9 in the apoptosis regulation mechanism (35). This research showed that FASN-mediated anti-apoptosis was crucial to the formation of HCC and that PCSK9 facilitated this proliferation (26, 30, 35). Nowadays, it has been found that using lipopolysaccharide (LPS) causes PCSK9 expression to be down-regulated while the expressions of SREBP2, HMGCR, and LDL-R are up-regulated (Figure 2) (32). In malignancies, PCSK9 expression was associated with poor outcomes in patients with HCC.
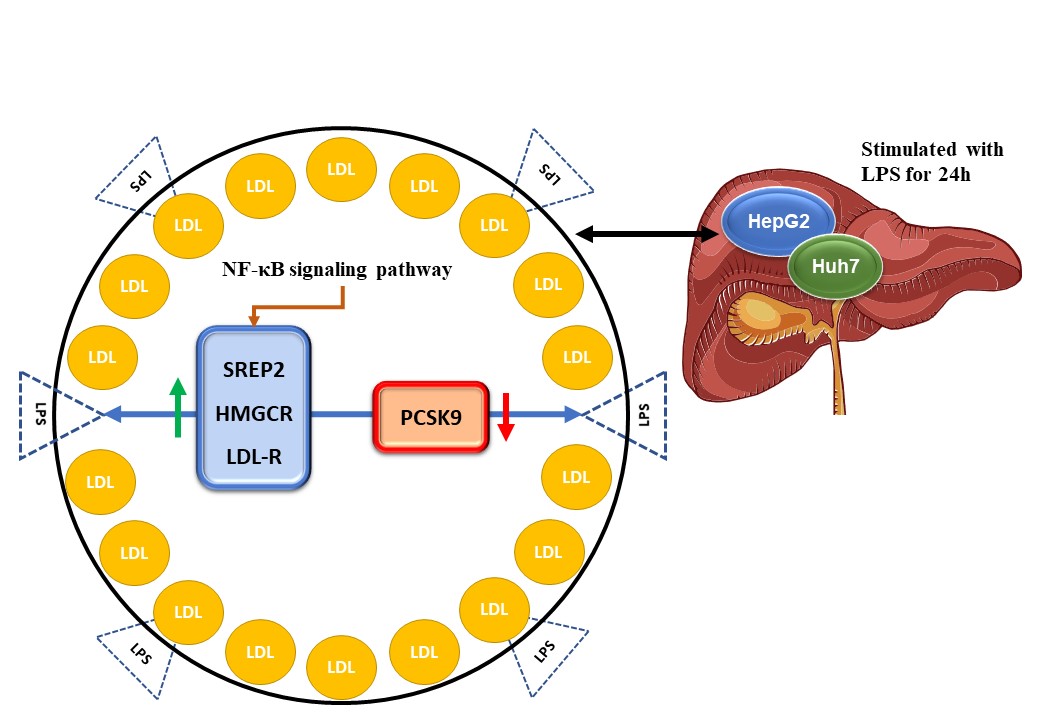
Fig. 2.The schematic representation effect of LPS on PCSK9 in HCC (32, 36, 37).
LPS is used for 24 hours to activate HepG2 and Huh7 cell lines as human HCC cells and significantly enhances intracellular cholesterol levels by increasing the expression of SREBP2, HMGCR, and LDL-R while down-regulating the expression of PCSK9 (Down-regulated way). Surprisingly, these results relied on the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling pathway.
1.2. PCSK9 and Lung Cancer
Non-small cell lung cancer (NSCLC) is the most common lung cancer type, with more than 80% of all occurrences (38). A study has shown that PCSK9 levels in lung tumor samples are considerably lower than in normal surrounding tissue (32). By targeting adenocarcinoma in NSCLC therapy, human alveolar basal epithelial cells (A549, a cell line of human lung adenocarcinoma) were transfected with PCSK9 siRNA, and it was discovered that PCSK9 siRNA could inhibit proliferation and increase apoptosis of A549 cells by inducing endoplasmic reticulum (ER) stress and mitochondrial dysfunction (39, 40). Patients with low degrees of PCSK9 had an excellent response to ICI therapy, which has enabled the development of PCSK9-based absolute biomarkers or scientific drugs (39-41).
1.3. PCSK9 and Breast Cancer
Breast cancer is a common disease in women worldwide, with 1.5 million women diagnosed each year (42). Pseurotin A (PS) is a special spiro heterocyclic γ-lactam alkaloid from the fungal culture of Pseudeurotium ovalis (38). Many studies have found that PS inhibits PCSK9 secretion in breast cancer by targeting BALB/c mice (38). While lipids were related to lung and colorectal cancer risks, no association was found between lipids and the histological characteristics of breast cancer tumors (43, 44). But still, in 2008 research by Shah et al., indicates that triglycerides may be negatively related to breast cancer risk, whereas HDL-C might protect postmenopausal women from breast cancer (45, 46). Coexisting physiological factors, such as an underlying metabolic syndrome, post-menopausal state, or chemotherapy, might impact the amounts of circulating lipids, potentially obscuring the relationship between lipid profile and breast cancer prognosis (42-44, 46). As a result of these studies, inhibiting PCSK9 may even improve breast cancer behavior and hold promise as a diagnostic and prognostic biomarker.
1.4. PCSK9 and Prostate Cancer
Prostate cancer (PC) is the second most commonly diagnosed cancer in men and the fifth cause of death globally (47, 48). PCSK9 siRNA therapy dramatically improved cell survival, reduced apoptosis, and protected lymph node carcinoma of the prostate (LnCap) against cell damage by increasing the expression of cytochrome C (cyto C), B-cell leukemia/lymphoma 2 (Bcl-2), and Bcl-2 associated X protein (49, 50). According to the convincing evidence derived from large-scale genetic data, the therapeutic suppression of lipid-lowering medications targeting PCSK9 may lessen the incidence of prostate cancer (51). Recent research shows that genetically mediated regulation of PCSK9 is significantly linked to a decreased risk of both overall and early-onset prostate cancer, perhaps through a mechanism involving the reduction of Lp(a) levels (47, 50-52).
| Cancer Name | Most Influential Factor | Expression Level | Refs |
|---|---|---|---|
| Hepatocellular carcinoma | GSTP1, JNK signaling pathway, and HepG2 | Upregulated | (1, 27, 30, 38) |
| Lung Cancer | Adenocarcinoma human alveolar basal epithelial cells (A549) | Downregulated | (21, 40) |
| Breast Cancer | Pseurotin A (PS) – BALB/c | Upregulated | (38, 44, 46) |
| Prostate Cancer | Cyto C, Bcl-2, and Bax-LnCap | Downregulated | (32, 47, 50, 51) |
2. PCSK9 and Interaction with MHC-I
Major histocompatibility complex 1 (MHC-I) is synthesized by ER, then assembled with beta 2-microglobulin and stored by the ER until loaded with antigenic peptides (53). Within the cytosol, MHC-I molecules attach to the antigens and infectious agents such as viral particles and tumor-derived molecules (54). The expression of MHC-I proteins on cancer cells increases when PCSK9 is inhibited, resulting in a massive influx of cytotoxic T lymphocytes (Figure 1) (23). T Lymphocytes and the effector chemicals are essential for regulating spontaneous, induced, or transplanted immunity (55). Xinjian Liu et al., demonstrated that CD8+, CD4+ T helper cells (Th), T cells, and natural killer cells (NKCs) were significantly increased in PCSK9-deficient tumors in a flow cytometry study (23, 56). According to their findings, PCSK9-deficient tumor cells have many T-cell receptors (TCRs) and a wide diversity of mature T-cells (23). As a result of MHC interaction with TCRs, other stimuli trigger the immune response. T lymphocytes and other immune cells, such as macrophages, eliminate MHC-I positive or heterogeneous tumor cells (5). This is a novel finding regarding how PCSK9 regulates cell surface MHC-I and thus influences intra-tumoral immune infiltration. Thus, it is possible that neutralizing PCSK9 encourages intra-tumoral T-cell infiltration and makes tumors more susceptible to immune checkpoint therapy (Figure 3) (57).
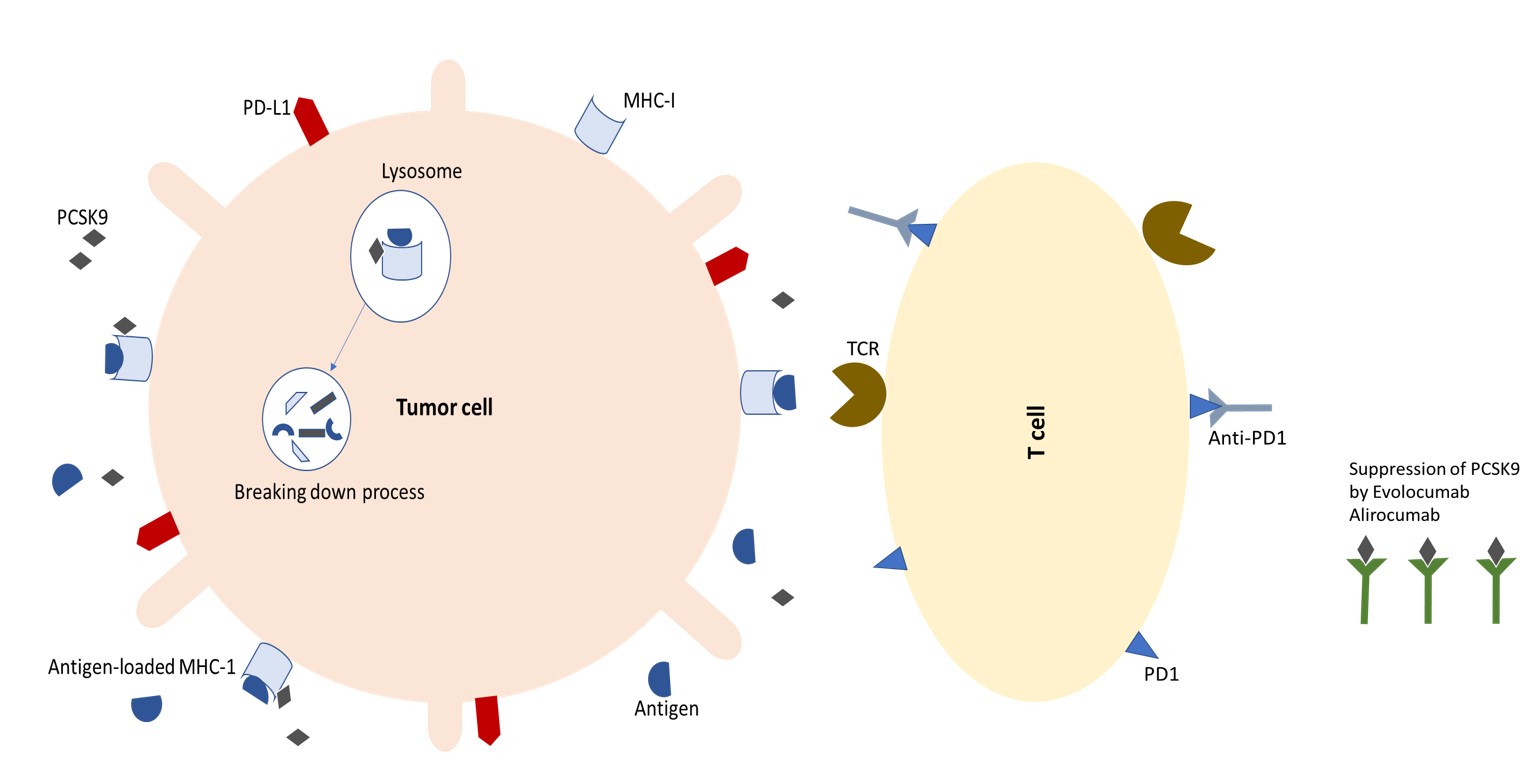
Fig. 3.T cells attack tumor cells by binding to antigens on MHC-I molecules on their surfaces, initiating the adaptive immune response. On the other hand, with the connection of PD1 and anti-PD1 antibodies, the possibility of immunosuppression is neutralized. Both of these functions increase the anti-cancer effect. As a result of PCSK9 inhibition, T cells infiltrate the tumor, making it susceptible to immune checkpoint therapy (23, 58).
The immune response requires two signals to activate T cells; triggered by B7.1/CD80 or B7.2/CD86 interacting with naive T cells and the TCR (59, 60). CTLA-4 inhibits T cell activity by binding to B7 and is being studied as a potential prognosis marker in cancer treatment (15). PD-L1 is a ligand for the immune checkpoint PD-1, and its interaction negatively regulates T cell activation (61, 62). Antibodies targeting PD-1 or PD-L1 have become the new standard cancer treatment (15, 63). Increased CTLA-4 and PD-1/PD-L1 expression is associated with poorer overall survival (62). Tumor-immune evasion commonly involves decreased MHC-I expression and increased immune checkpoint ligands on the cell surface (64, 65). PD-L1 expression in tumors can serve as a biomarker for the treatments inhibiting this molecule, and a higher level of MHC-I expression indicates a potential response to the immune checkpoint therapy (5, 66, 67).
Now, it is possible to understand how immune checkpoints affect tumor development and how PCSK9 plays a role in this way (68). The immune checkpoints are inhibitory pathways vital for the self-tolerance and maintenance of collateral tissue protection by modulating the immune responses and their length (68). Thus, it is clear that tumors choose immune checkpoint pathways as a primary immune resistance mechanism, especially against T cells specific to the tumor antigens (53); therefore, they may be a potential target for cancer immunotherapy. In immunotherapies, the patient's immune system is used to fight cancer (23). They may overcome resistance mechanisms associated with other medicines by directly targeting the immune system (69). The balance between tumor cells and the immune system allows the tumor to grow uncontrollably and shift in favor of the tumor (46). The emergence of tumor cells with decreased immunogenicity is an example of this escape system, which dampens the anti-tumor immune response for tumor elimination (70). The ICIs have been used for decades to reactivate the immune system by inactivating checkpoint inhibitory proteins on cancer cells or T cells and to help the immune system detect and attack cancer cells (71). PD-1, CTLA-4, lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT), and B- and T-lymphocyte attenuator (BTLA) (Table 2) are few examples of inhibitory immune checkpoint receptors, which were discovered and studied in cancer (60). Treatment of different cancers requires selectively targeting immune checkpoints with specially designed checkpoint-blocking antibodies (like CTLA-4 and PD-1) (63). Thus, PCSK9 inhibition has been proposed as a potential strategy to improve immune checkpoint treatment by inhibiting proteins that suppress checkpoint signaling pathways in T cells and improving their reaction to tumor cells (20).
| ICRs | Expressed on | A mechanism on T cell | Marker For | Ref |
|---|---|---|---|---|
| PD-1 | All T cells during activation. | Connection by PD-L1 leads to rapid termination of TCR intracellular signaling and inhibition of T cell proliferation. | - Angioimmunoblastic lymphoma- Downregulates immune responses | (53) |
| CTLA-4 | All T cells during activation. | Decreasing the function of T cells. | Downregulates immune responses | (72) |
| LAG-3 | Activated T cells, natural killer cells, B cells, and plasmacytoid dendritic cells | Encouraging differentiation into T regulatory cells | Offensive progression in different human tumors such as;- Melanoma,- Hodgkin's lymphoma,- Chronic lymphocytic leukemia,- Colorectal cancer,- Ovarian cancer, etc. | (73) |
| TIM-3 | Interferon-γ-producing CD4+ and CD8+ T cells. Monocytes | Suppress T-cell responses upon interaction with their ligand(s). | Activation marker of macrophages and an inhibitor of macrophage activity | (74) |
| TIGIT | Activated T cells are also found in NK cells | Binds to T cell receptors and triggers direct inhibitory signals. | T-Cell lethargy in Liver Cancer | (75) |
| BTLA | CD4/CD8 single-positive T-cells | BTLA inhibits T-cell reactions and cytokine production | Demonstrate putative permissive activation state of B cell subtypes in healthy blood donors. | (76) |
Conclusion
The present study shows PCSK9 functions through many mechanisms, including regulation of several cellular receptors, controlling circulating LDL, and apoptosis pathways, and regulation of the immune response to the tumor cells (71). The discovery that PCSK9 modulates cell surface MHC-I levels and intratumoral immune infiltration is novel in terms of mechanism. When PCSK9 is inhibited, various malignancies respond better to immune checkpoint therapy (64). Furthermore, our findings show that a combination of Alirocumab and Evolocumab significantly may reduce cholesterol levels by inhibiting PCSK9 (32). Based on previous studies, ICIs are strongly associated with activated T cells. Several immune checkpoints, including CTLA-4, PD-1, LAG3, and TIM3, inhibit immune system activity; blocking them triggers immune responses against cancerous cells (60). Finally, more individualized tumor genetics-based immune checkpoint combination approaches (such as PCSK9) must be researched. Despite numerous challenges, there is optimism that checkpoint inhibitors are paving the way to a new cancer treatment era.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
None.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
None.
References
- Bhat M, Skill N, Marcus V, Deschenes M, Tan X, Bouteaud J. Decreased PCSK9 expression in human hepatocellular carcinoma. BMC Gastroenterology. 2015; 15(1):1-10.
- Catapano AL, Pirillo A, Norata GD. New pharmacological approaches to target PCSK9. Curr Atheroscler Rep. 2020; 22(7):1-8.
- Bittner V. Pleiotropic effects of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors? Circulation. 2016; 134(22):1695-6.
- Cui C-J, Li S, Li J-J. PCSK9 and its modulation. Clin Chim Acta.. 2015; 440:79-86.
- Yuan J, Cai T, Zheng X, Ren Y, Qi J, Lu X. Potentiating CD8+ T cell antitumor activity by inhibiting PCSK9 to promote LDLR-mediated TCR recycling and signaling. Protein Cell. 2021; 12(4):240-60.
- Caruso S, Giudicissi R, Mariatti M, Cantafio S, Paroli GM, Scatizzi M. Laparoscopic vs Open Gastrectomy for Locally Advanced Gastric Cancer: A Propensity Score-Matched Retrospective Case-Control Study. Curr Oncol. 2022; 29(3):1840-65.
- Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017; 376(1):41-51.
- Javid H, Karimi-Shahri M, Khorramdel M, Sathyapalan T, Afshari A, Sahebkar A. Probiotics as an adjuvant for management of gastrointestinal cancers through their anti-inflammatory effects: a mechanistic review. Curr Med Chem. 2022.
- Karimi-Shahri M, Javid H, Yazdani S, Hashemy SI. Mesenchymal stem cells in cancer therapy; the art of harnessing a foe to a friend. Iran J Basic Med Sci. 2021; 24(10):1307.
- Karimi-Shahri M, Khorramdel M, Zarei S, Attarian F, Hashemian P, Javid H. Glioblastoma, an opportunity T cell trafficking could bring for the treatment. Mol Biol Rep. 2022;1-13.
- Javid H, Ghahremanloo A, Afshari AR, Salek R, Hashemy SI. The Emerging Role of Neurokinin-1 Receptor Blockade Using Aprepitant in the Redox System of Esophageal Squamous Cell Carcinoma. Int J Pept Res Ther. 2022; 28(3):1-13.
- Rezaei S, Assaran Darban R, Javid H, Hashemy SI. The therapeutic potential of aprepitant in glioblastoma cancer cells through redox modification. BioMed Res Int. 2022; 2022
- Mehrabani N, Vaezi Kakhki MR, Javid H, Ebrahimi S, Hashemy SI. The SP/NK1R System-Mediated ROS Generation in GBM Cells through Inhibiting Glutaredoxin Protein. Neurol Res Int. 2021; 2021
- Xu Y, Poggio M, Jin HY, Shi Z, Forester CM, Wang Y. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat Med. 2019; 25(2):301-11.
- Saleh R, Toor SM, Sasidharan Nair V, Elkord E. Role of epigenetic modifications in inhibitory immune checkpoints in cancer development and progression. Front Immunol.. 2020; 11:1469.
- Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA‐4 and PD‐1 blockade as cancer immunotherapy. J Leukoc Biol. 2013; 94(1):25-39.
- Ghosh C, Luong G, Sun Y. A snapshot of the PD-1/PD-L1 pathway. J Cancer. 2021; 12(9):2735.
- Mabeza RM, Lee C, Verma A, Park MG, Darbinian K, Darbinian S. Factors and Outcomes Associated With Venous Thromboembolism Following Bariatric Surgery. Am Surg. 2022; 88(10):2525-30.
- Ledford H. Melanoma drug wins US approval. Nature. 2011; 471(7340):561.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12(4):252-64.
- Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017; 50:2.
- Webb ES, Liu P, Baleeiro R, Lemoine NR, Yuan M, Wang Y. Immune checkpoint inhibitors in cancer therapy. J Biomed Res. 2018; 32(5):317.
- Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J. PCSK9 inhibition potentiates cancer immune checkpoint therapy. Nature. 2020; 588(7839):693 .
- Chaudhary R, Garg J, Shah N, Sumner A. PCSK9 inhibitors: a new era of lipid lowering therapy. World J Cardiol. 2017; 9(2):76.
- Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am College Cardiol. 2015; 65(24):2638-51.
- Alannan M, Seidah NG, Merched AJ. PCSK9 in Liver Cancers at the Crossroads between Lipid Metabolism and Immunity. Cells. 2022; 11(24):4132.
- Athavale D, Chouhan S, Pandey V, Mayengbam SS, Singh S, Bhat MK. Hepatocellular carcinoma-associated hypercholesterolemia: involvement of proprotein-convertase-subtilisin-kexin type-9 (PCSK9). Cancer Metabol. 2018; 6(1):1-16.
- He M, Hu J, Fang T, Tang W, Lv B, Yang B. Protein convertase subtilisin/Kexin type 9 inhibits hepatocellular carcinoma growth by interacting with GSTP1 and suppressing the JNK signaling pathway. Cancer Biol Med. 2022; 19(1):90.
- Bonaventura A, Vecchié A, Ruscica M, Grossi F, Dentali F. PCSK9 as a new player in cancer: New opportunity or red herring? Curr Med Chem. 2022; 29(6):960-9.
- Zhang S-Z, Zhu X-D, Feng L-H, Li X-L, Liu X-F, Sun H-C. PCSK9 promotes tumor growth by inhibiting tumor cell apoptosis in hepatocellular carcinoma. Exp Hematol Oncol. 2021; 10(1):1-11.
- Athavale D, Chouhan S, Pandey V, Mayengbam SS, Singh S, Bhat MK. Hepatocellular carcinoma-associated hypercholesterolemia: involvement of proprotein-convertase-subtilisin-kexin type-9 (PCSK9). Cancer Metabol.. 2018; 6:1-16.
- Bhattacharya A, Chowdhury A, Chaudhury K, Shukla PC. Proprotein convertase subtilisin/kexin type 9 (PCSK9): A potential multifaceted player in cancer. Biochim Biophys Acta (BBA)-Rev Cancer. 2021; 1876(1):188581.
- Ruscica M, Ferri N, Macchi C, Meroni M, Lanti C, Ricci C. Liver fat accumulation is associated with circulating PCSK9. Ann Med. 2016; 48(5):384-91.
- Alannan M, Trézéguet V, Amoêdo ND, Rossignol R, Mahfouf W, Rezvani HR. Rewiring Lipid Metabolism by Targeting PCSK9 and HMGCR to Treat Liver Cancer. Cancers. 2022; 15(1):3.
- Paquette M, Gauthier D, Chamberland A, Prat A, Rolfe EDL, Rasmussen JJ. Circulating PCSK9 is associated with liver biomarkers and hepatic steatosis. Clin Biochem.. 2020; 77:20-5.
- Ragusa R, Basta G, Neglia D, De Caterina R, Del Turco S, Caselli C. PCSK9 and atherosclerosis: Looking beyond LDL regulation. Eur J Clin Invest. 2021; 51(4):e13459.
- Feder S, Wiest R, Weiss TS, Aslanidis C, Schacherer D, Krautbauer S. Proprotein convertase subtilisin/kexin type 9 (PCSK9) levels are not associated with severity of liver disease and are inversely related to cholesterol in a cohort of thirty eight patients with liver cirrhosis. Lipids Health Dis.. 2021; 20:1-14.
- Mahboobnia K, Pirro M, Marini E, Grignani F, Bezsonov EE, Jamialahmadi T. PCSK9 and cancer: Rethinking the link. Biomed Pharmacother.. 2021; 140:111758.
- Bonaventura A, Grossi F, Carbone F, Vecchié A, Minetti S, Bardi N. Serum PCSK9 levels at the second nivolumab cycle predict overall survival in elderly patients with NSCLC: a pilot study. Cancer Immunol Immunother. 2019; 68(8):1351-8.
- Xie M, Yu X, Chu X, Xie H, Zhou J, Zhao J. Low baseline plasma PCSK9 level is associated with good clinical outcomes of immune checkpoint inhibitors in advanced non‐small cell lung cancer. Thoracic Cancer. 2022; 13(3):353-60.
- Luo X, Xu JG, Wang Z, Wang X, Zhu Q, Zhao J. Bioinformatics identification of key genes for the development and prognosis of lung adenocarcinoma. INQUIRY: J Health Care Organization Provis Financing.. 2022; 59:00469580221096259.
- Sun Y-S, Zhao Z, Yang Z-N, Xu F, Lu H-J, Zhu Z-Y. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017; 13(11):1387.
- Momtazi-Borojeni AA, Nik ME, Jaafari MR, Banach M, Sahebkar A. Effects of immunization against PCSK9 in an experimental model of breast cancer. Arch Med Sci. 2019; 15(3):570-9.
- Momtazi-Borojeni AA, Nik ME, Jaafari MR, Banach M, Sahebkar A. Effects of immunization against PCSK9 in an experimental model of breast cancer. Arch Med Sci. 2019; 15(3):570.
- Shah FD, Shukla SN, Shah PM, Patel HR, Patel PS. Significance of alterations in plasma lipid profile levels in breast cancer. Integr Cancer Ther. 2008; 7(1):33-41.
- Wong Chong E, Joncas F-H, Seidah NG, Calon F, Diorio C, Gangloff A. Circulating levels of PCSK9, ANGPTL3 and Lp (a) in stage III breast cancers. BMC Cancer. 2022; 22(1):1-12.
- Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019; 10(2):63.
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019; 10(1):10.
- Ng ACT, Delgado V, Borlaug BA, Bax JJ. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol. 2021; 18(4):291-304.
- Gan S-S, Ye J-Q, Wang L, Qu F-J, Chu C-M, Tian Y-J. Inhibition of PCSK9 protects against radiation-induced damage of prostate cancer cells. OncoTarg Ther.. 2017; 10:2139.
- Fang S, Yarmolinsky J, Gill D, Bull CJ, Perks CM, Consortium P. Association between genetically proxied PCSK9 inhibition and prostate cancer risk: A Mendelian randomisation study. Plos Med. 2023; 20(1):e1003988.
- Abdelwahed KS, Siddique AB, Qusa MH, King JA, Souid S, Abd Elmageed ZY. PCSK9 Axis-targeting pseurotin A as a novel prostate cancer recurrence suppressor lead. ACS Pharmacol Transl Sci. 2021; 4(6):1771-81.
- Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. 2020; 588(7839):693-8.
- Almeida CR, Ferreira BH, Duarte IF. Targeting PCSK9: a promising adjuvant strategy in cancer immunotherapy. Signal Transduct Target Ther. 2021; 6(1):111 .
- Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol.. 2018; 62:29-39.
- Crunkhorn S. Blocking PCSK9 enhances immune checkpoint therapy. Nat Rev Drug Discov. 2021; 20(1):20-1.
- Volpe M, Patrono C. PCSK9 inhibition: Not just LDL-Cholesterol knock down: A glimmer for cancer. Eur Heart J. 2021.
- Jin S, Sun Y, Liang X, Gu X, Ning J, Xu Y. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct Target Ther. 2022; 7(1):39.
- Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol.. 2021; 16:223-49.
- He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020; 30(8):660-9.
- Armand P, Lesokhin A, Borrello I, Timmerman J, Gutierrez M, Zhu L. A phase 1b study of dual PD-1 and CTLA-4 or KIR blockade in patients with relapsed/refractory lymphoid malignancies. Leukemia. 2021; 35(3):777-86.
- Bewersdorf JP, Shallis RM, Zeidan AM. Immune checkpoint inhibition in myeloid malignancies: Moving beyond the PD-1/PD-L1 and CTLA-4 pathways. Blood Rev.. 2021; 45:100709.
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015; 33(17):1974.
- Erdogdu IH. MHC class 1 and PDL-1 status of primary tumor and lymph node metastatic tumor tissue in gastric cancers. Gastroenterol Res Pract.. 2019; 2019:4785098.
- Kahlmeyer A, Stöhr CG, Hartmann A, Goebell PJ, Wullich B, Wach S. Expression of PD-1 and CTLA-4 are negative prognostic markers in renal cell carcinoma. J Clin Med. 2019; 8(5):743.
- Hutarew G. PD-L1 testing, fit for routine evaluation? From a patholo gist's point of view. Memo – Mag Eur Med Oncol. 2016; 9(4):201-6.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366(26):2443-54.
- Chen Y-S, Shen C-R. Immune checkpoint blockade therapy: the 2014 Tang Prize in Biopharmaceutical Science. Biomed J. 2015; 38(1):5-8.
- Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann the New York Acad Sci. 2013; 1291(1):1-13.
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002; 3(11):991-8.
- Patrono C, Volpe M. Oxford University Press; 2021.
- Mittal AK, Chaturvedi NK, Rohlfsen RA, Gupta P, Joshi AD, Hegde GV. Role of CTLA4 in the proliferation and survival of chronic lymphocytic leukemia. PloS One. 2013; 8(8):e70352.
- Zhang Q, Chikina M, Szymczak-Workman AL, Horne W, Kolls JK, Vignali KM. LAG3 limits regulatory T cell proliferation and function in autoimmune diabetes. Science Immunol. 2017; 2:9.
- Liu Z, Xiang C, Han M, Meng N, Luo J, Fu R. Study on Tim3 Regulation of Multiple Myeloma Cell Proliferation via NF-κB Signal Pathways. Frontiers Oncol.. 2020; 10:584530.
- Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011; 186(3):1338-42.
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003; 4(7):670-9.

