Document Type : Original Research
Authors
1 Department of Clinical Biochemistry, Medical School, Kermanshah University of Medical Sciences, Kermanshah, Iran
2 Medical Biology Research Center, Research Institute for Health Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran
3 Department of Dermatology, Medical School, Kermanshah University of Medical Sciences, Kermanshah, Iran
4 Fertility and Infertility Research Center, Medical School, Kermanshah University of Medical Sciences, Kermanshah, Iran
5 Department of Internal Medicine, Medical School, Kermanshah University of Medical Sciences, Kermanshah, Iran
Highlights
✅We found the absence of an association between IGF-1 and MTHFR polymorphisms with the risk of AV. However, increased insulin, IGF-1, and HOMA levels in AV patients indicated the effect of insulin and insulin resistance in the risk of AV and its severity.
Keywords
Subjects
Acne vulgaris (AV) is a chronic inflammatory skin disease, which is particularly common during adolescence and in young adults. This disease impairs various aspects of life quality (1). Clinical features of AV include seborrhea (excess grease), inflammation, abnormal follicular keratinization, and various degrees of scarring. The AV appears to be influenced by different factors, such as hereditary and genetics, nourishing, and environmental factors (2). Since AV reduces self-reliance in most teenage patients, its diagnosis and treatment are very important.
The insulin hormone has various roles in body metabolism. The insulin gene is located at the end of the short arm of chromosome 11, synthesized in β cells of the pancreas, and released in response to blood glucose to decrease it (3).
The gene of insulin-like growth factor-1 (IGF-1) is located on chromosome 12 and with similar structure to insulin. Insulin, growth hormone, and IGF-1 are effective factors in stimulating AV. However, IGF-1 is a stronger stimulant for sebum production and development of AV compared to insulin. The insulin and IGF-1 signaling is increased during puberty by a carbohydrate-rich diet, which may prompt the AV (4). The 5, 10-methylenetetrahydrofolate reductase (MTHFR) plays a key role in folate metabolism and synthesis of DNA. The C677T polymorphism of the MTHFR gene results in an alanine to valine alteration at position 222 of the polypeptide, which reduces the activity of the MTHFR (5). Severe MTHFR deficiency is associated with hyperhomocysteinemia, and the presence of MTHFR C677T polymorphism due to decreased MTHFR activity increases the susceptibility to the onset and progression of diabetic nephropathy in type 2 diabetes mellitus (6).
The present study aimed to investigate the possible association between variants of the MTHFR and IGF-1 genes, as well as the levels of IGF-1, insulin, and homeostasis model assessment (HOMA) with the risk and severity of AV in a population from Kermanshah province in Western Iran.
Sample
A total of 150 patients with mild-, moderate-, and severe-AV and 148 healthy individuals without symptoms and history of AV were studied in this research. Basic information of each individual, including the age, sex, weight, height, age of disease onset, period of disease, family history of AV, severity of AV symptoms, and blood pressure were collected. All individuals were from Kermanshah province in Western Iran. AV grading area was defined according to the Global Acne Grading System, which divides the face, chest, and upper back into six areas, and assigns a number to each area based on the surface area, distribution, and density of pilosebaceous units (7).
The study was approved by the Ethics Committee of Kermanshah University of Medical Sciences and was in accordance with the principles of the Declaration of Helsinki II.
Biochemical and Hormone Assay
The blood sample was collected after overnight fasting. The levels of serum fasting blood sugar (FBS) triglyceride (TG), cholesterol, low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C) were measured by using Bionic Diagnostic Kits (Iran) on Mindray BS-480 Chemistry Analyzer (China). Insulin and IGF-1 were evaluated by Monobind and Diametra hormone ELISA kits (made in USA and Italy respectively). The serum level of estradiol, in the mid-follicular phase of the menstrual cycle, was measured via the chemiluminescent method by using the Abbott Architect i1000 (Abbott Laboratory, USA).
Also, the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows:
DNA Analysis
Genomic DNA was extracted from peripheral leukocytes of ethylenediaminetetraacetic acid (EDTA)-treated whole blood by the phenol-chloroform method (8). The G>A variant of the IGF-1 gene (rs6214) was amplified via polymerase chain reaction (PCR). The obtained PCR product with190-bp was digested by Hin 1 II (NlaIII) at 37˚C for overnight; then, fragments were separated by 3% agarose gel (9).
The C677T polymorphism of the MTHFR gene (rs1801133) was amplified via PCR and subsequent digestion with Hinf I, as previously described (10).
Statistical Analysis
The statistical package for social sciences (SPSS) 16 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The frequencies of alleles of both polymorphisms were calculated by the chromosome counting method. The χ2 test was used to calculate the significance of difference in the frequencies of genotypes and alleles of both polymorphisms between patient and control groups A two-tailed student’s t-test and analysis of variance (ANOVA) were used to compare quantitative data. The P-value of less than 5% was considered as a statistically significant level.
The biochemical characteristics of patients and controls are demonstrated in Table 1. As indicated in Table 1, both patient and control groups were age-matched (P=0.29). The serum level of insulin in AV patients (16.7±26 µU/mL) was significantly higher than in normal ones (5.48±5.5 µU/mL, P<0.001). However, a near to significant difference, between IGF-1 serum levels of cases (179.8±72 µg/L) and controls (164.6±63 µg/L), was found (P=0.056). The FBS level was 80.0±10.9 mg/dL in all AV patients compared to 77.5±17.0 mg/dL in controls (P=0.94). HOMA-IR was significantly higher in AV patients (3.54±5.6, P<0.001) than in controls (1.16±1.4). The HDL-C serum level was significantly higher in patients (48.5±12.9 mg/dL) than in controls (41.2±10.5 mg/dL, P<0.001). Parameters were compared in both sexes between the patients and the controls (Table 2). Insulin and HOMA-IR values were significantly higher in female patients than in female controls. Significantly higher levels of FBS, total cholesterol, and LDL-C were detected in female patients than in female controls. However, the level of estradiol was significantly lower in female patients (88.4±81.9 pg/mL) than in female controls (118±86.9 pg/mL, P=0.004) (Table 2). As indicated in Table 2, among males, only the cholesterol level was significantly higher in controls than in patients.
Table 1. Characteristics of AV patients and controls
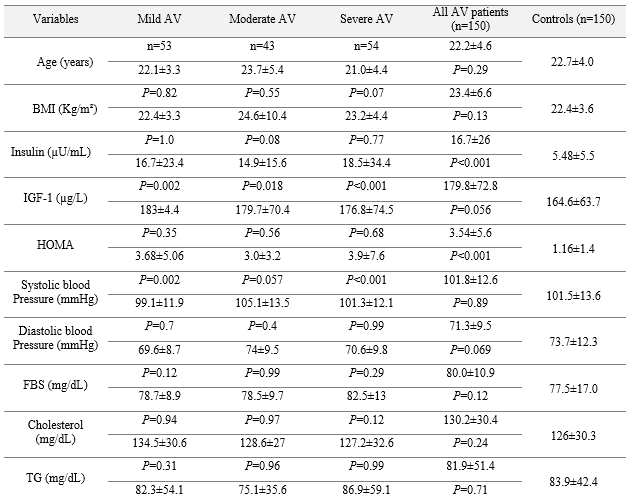

Table 2. Characteristics of males and females in both groups of patients and controls


Table 3. Comparison of the IGF-1 genotype and allele frequencies between patients with mild-, moderate-, and severe-AV and controls

Table 4. Comparison of the frequency of MTHFR genotypes and alleles between patients with mild-, moderate-, and severe-AV and controls

Various factors including genetic background, nutrition and environment affect the incidence of AV. Previously we examined the role of some genetic variants and also hormonal factors and lipid profile in susceptibility to AV (2,7).
In the present study, the higher serum levels of insulin, HOMA, and HDL-C were detected in all patients with AV compared to controls. The gender-specific differences were observed in the biochemical characteristics of patients and controls. In females, increased insulin, HOMA, total cholesterol, and LDL-C levels, as well as decreased estradiol levels, were associated with the presence of AV. Changes in lipid profile can be considered in the treatment of female patients with moderate to severe AV (11). On the other hand, estradiol, the major female estrogenic hormone, may prevent the production of androgen and thus decrease the growth of the sebaceous gland and thereby decrease sebaceous gland growth (12). Therefore, the increased level of estradiol reduces the risk of AV and its severity. In males, just increased cholesterol was associated with AV. In one available study, among males with AV, it was shown that HDL-C and insulin could be involved in AV development (13).
In our study, the serum level of insulin was significantly higher in patients than in healthy individuals. The comparison between different types of AV showed the absence of an association between the insulin level and the severity of AV. However, insulin and HOMA were associated with the risk of AV in females but not in males. Some endocrine diseases can be associated with the AV risk. In females with AV, similar to patients with polycystic ovary syndrome (PCOS), insulin resistance is known to be a risk factor, which changes the level of androgens (13). In patients with PCOS, insulin resistance and hyperinsulinemia play an important role in the molecular mechanisms involved in ovarian androgenic hyper-secretion typical of PCOS (14).
On the other hand, insulin and IGF-1 cause hormonal changes in adrenal and gonadal androgen synthesis (15). Changes in androgens and hormonal mediators are effective in the production of sebum and consequently AV susceptibility (16). Several studies presented different results about the relationship between the AV risk and the IGF-1 level. Behrangi et al. found that the mean serum level of IGF-1 was significantly associated with AV (17). However, there was no association between the severity of AV and the IGF-1 level. In another study, IGF-1 had a stronger effect on acne in women compared to men, but androgens played a greater role in acne among men (18). According to both studies, gender difference can influence the IGF-1 level in patients with AV. In our study, although the serum level of IGF-1 was higher in patients compared to controls, its difference was near to significant. Thus, the level of IGF-1 may almost be a possible pathogenic factor in AV.
In the human body, the IGF-1 gene encodes a protein that has a similar insulin function and structure. It is a significant contributor to cellular growth and development, protein translation, differentiation, metabolism, apoptosis, etc. Also, this gene may influence the androgen secretion and thus be effective in the pathogenesis of AV (19). It seems that among the Turkish population IGF-1 (cytosine adenosine, CA) 19 polymorphism is associated with the predisposition to AV (20). Also, Rahaman et al. found an association between IGF-1 (cytosine adenosine) 19 polymorphism with AV and its severity (21). In the current study, we investigated another polymorphism of IGF-1 G>A and found the absence of an association between this polymorphism and the risk of AV.
The MTHFR polymorphism was also evaluated by considering its key role in the pathway of folate metabolism and DNA, RNA, and protein methylation. A frequency of 27.85% for the MTHFR T allele has been reported among healthy individuals from Western Iran (22). In the present study, the MTHFR C677T polymorphism had no relationship with the AV susceptibility. However, among women, our study detected that in the presence of MTHFR T allele the levels of FBS and LDL-C were higher and the level of estradiol was lower compared to the wild allele of MTHFR (C). The MTHFR C677T polymorphism has been associated with hyperlipidemia in Indian women with PCOS, and carriers of the MTHFR CT genotype had a significantly higher level of TG and cholesterol compared to carriers of the MTHFR CC genotype (23). However, among Korean women with PCOS, no relationship was reported between the MTHFR C677T polymorphism and the risk of PCOS (24).
Our study demonstrated that while there was no ERα expression detection, ERβ is probably expressed in the majority (if not all) of the glial tumors and its expression is conversely correlated with tumor grade. Previous and ongoing clinical trials have shown that ERβ agonists are well tolerated with acceptable side effects. Further trials are needed to clarify the potential role of ERβ agonists as a therapeutic option to enhance survival and quality of life of the patients with glial tumors.
This work was performed in partial fulfillment of the requirements for MSc degree of Sakineh Zinati and was financially supported by a grant from Vice Chancellor for Research of Kermanshah University of Medical Sciences, Kermanshah, Iran.
The authors declared that there is no conflict of interest regarding the publication of this article.
| Article View | 1,520 |
| PDF Download | 982 |