Document Type : Original Research
Authors
1 Department of Immunology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
2 Department of Obstetrics and Gynecology, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
Highlights
✅The decreased expression of polymorphic HLA class Ⅰ and Ⅱ by spermatozoa can be related to URSA occurrence.
Keywords
Subjects
Recurrent spontaneous abortion (RSA) is a common problem among couples and is defined as the occurrence of three or more clinically detectable pregnancy losses that usually occur before 20 weeks of gestation (1). Some causes of RSA are uterine anatomical defects and infections, chromosomal aberrations, hormonal disorders, and immunological abnormalities (1). In about 50% of RSA couples, the causes of abortion still remain unknown, which is so-called unexplained recurrent spontaneous abortion (URSA). It has been understood that URSA occurs as a result of immune system dysfunctions (1). To the best of our knowledge, every investigation, in different aspects of immunity in URSA patients, has demonstrated immune system irregularity in these patients (2-6). We suppose that a reason for this immune irregularity and dysfunction might be the alteration of antigens that stimulate female reproductive tract (FRT) immune response. Antigen type is considered to be one of the most important factors for the stimulation of appropriate immunity. As antigens, semen and spermatozoa stimulate FRT immune system. After entering the vagina, semen and spermatozoa induce innate and adaptive immune responses in the FRT. The result of this activated immunity is memory regulatory lymphocytes and memory effector lymphocytes formation, which is directed to paternal antigens (7-10). Therefore, any alteration in seminal and spermatozoa antigenicity can result in disturbed immune response and consequently pregnancy aberration (7-10). As an allogeneic cell, spermatozoon can induce strong immune responses. Of the antigens, which make the allogeneic cells as a strong inducer of the immune response, are polymorphic human leukocyte antigen (HLA) antigens. However, there is conflicting data about polymorphic HLA class Ⅰ and Ⅱ expression by spermatozoa (11-17). We recently showed that ejaculated spermatozoa express both HLA class Ⅰ and Ⅱ. Moreover, some studies demonstrated that HLA molecules have some critical roles in pregnancy and its complications (18-21).
Therefore, by considering the critical role of HLA antigens in reproduction and their main roles in the induction of immune response, we suppose that one of the most likely factors, which changes immunity in FRT after deposition of spermatozoa, is an abnormality in HLA antigen expression on spermatozoa. In other words, perhaps one of the causes, which disturbs immunity in URSA, is a change in HLA antigens. This change leads to unfavorable immune stimulation in FRT, and accordingly RSA occurs because there is no prepared condition for occurring and maintaining pregnancy. Given those aforementioned explanations, this paper aims to determine the HLA antigen expression level on spermatozoa in men whose wife suffer from URSA.
Subjects
A total of 15 URSA couples (the range of age is from 24 to 40 years for men and 21 to 38 years for women) with at least three consecutive first-trimester abortions and 20 normal couples (the range of age is from 26 to 42 years for men and 27 to 40 years for women) with at least one child were included in this case-control study. The husband of each woman had normal semen status according to the criteria from the World Health Organization (WHO). All male partners did not have any history of genital tract disorder, such as a history of infection, undescended testis, inguinoscrotal surgery, genital trauma, or testicular torsion. Informed consent was obtained from all subjects. Semen samples were collected by masturbation after 2-3 days of sexual abstinence. After liquefaction at room temperature for 60 min, sperm quality was assessed according to WHO standard guidelines (WHO, 1992). A sample with normal quality was selected to assess HLA expression. The protocol for this study was approved by the Ethics Committee of Isfahan University of Medical Sciences (Isfahan, Iran).
Sperm Purification
The AllGrad (LifeGlobal® Group, Canada) gradient technique was used for the purification of spermatozoa. Then, 2 mL of AllGrad Wash (LifeGlobal® Group, Canada) was added to the liquefied semen sample and centrifuged at 350 g for 10 min. Next, the pellet was re-suspended in 1 mL of AllGrad Wash. The gradient was prepared by pipetting 1 mL of AllGrad 90% gradient into the bottom of the centrifuge tube and then layered by 1 mL of AllGrad 45% gradient carefully on top of the lower layer, followed by 1 mL of the spermatozoa suspension. The tubes were centrifuged at 400 g for 18 min. The spermatozoa pellet at the bottom of the centrifuge tubes was washed and re-suspended in AllGrad Wash.
Flow Cytometry
To assess HLA class I and II expression on the surface of spermatozoa, we used the flow cytometry method. Two tubes were prepared for each sample containing 1×106 spermatozoa. One was incubated with phycoerythrin (PE) mouse anti-human HLA-ABC (BD Pharmingen, USA), and the other one was incubated with PE mouse anti-human HLA-DR at room temperature for 30 min. After two washes with AllGrad Wash (at 400 g for 5 min), tubes were run through flow cytometer (BD FACSCalibur, USA). Data from at least 100,000 events were collected by using forward scatter (a logarithmic amplifier) and side angle of light scatter (a logarithmic amplifier). Fluorescence data were obtained via the logarithmic amplifier. FlowJo VX software was used to analyze the data.
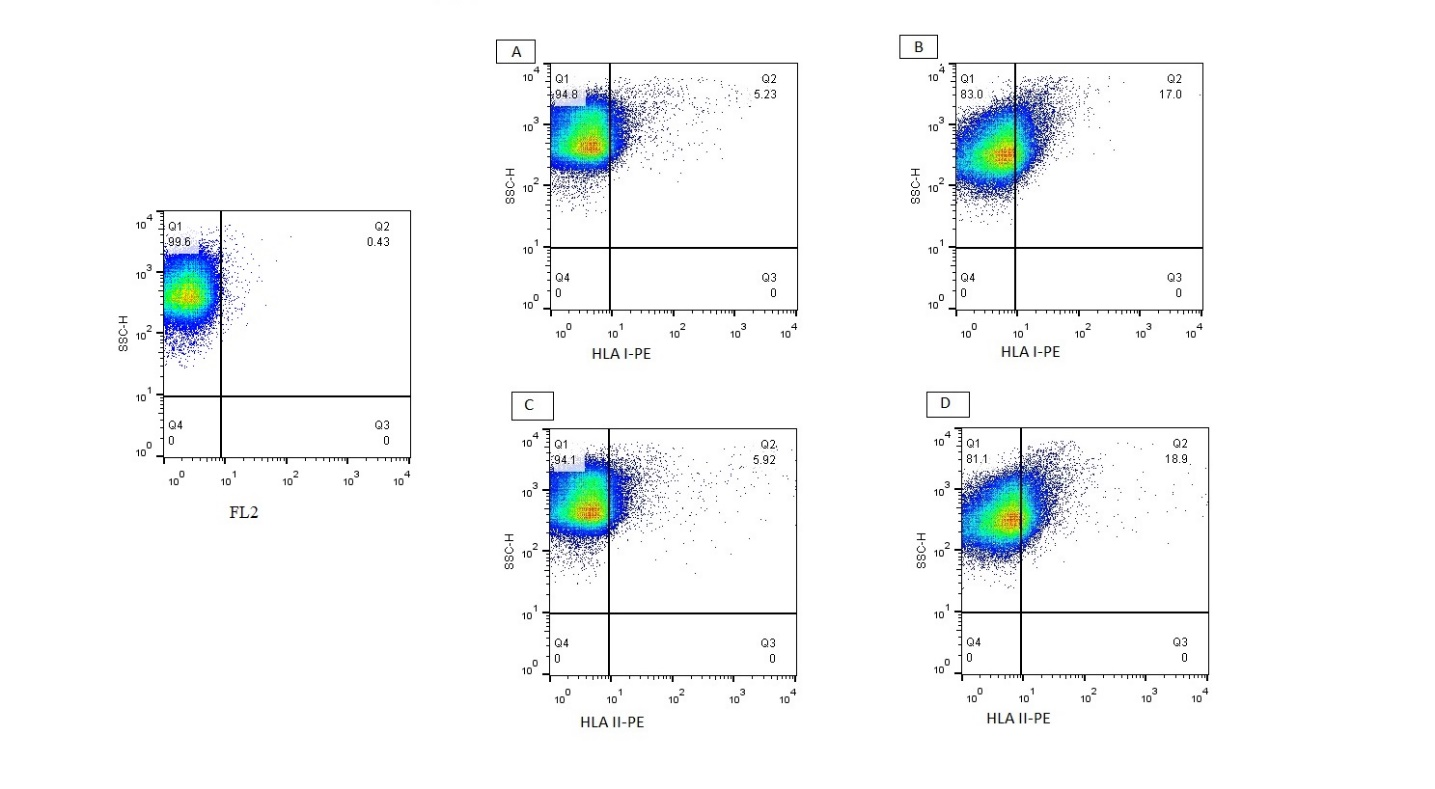
Fig. 1. Representative flow cytometry dot plots
A and C: flow cytometry dot plots in the RSA group; B and D: flow cytometry dot plots in the control group.
Statistical Analysis
The Student’s t-test was used to compare the differences between controls and URSA groups. Values were presented as mean ± standard deviation (SD). A P-value of
A flow cytometric assay was used to evaluate the HLA expression by spermatozoa (Figure 1). The results, which are presented in Figure 2, showed a significantly decreased mean percentage of the HLA class I and II expression by spermatozoa in the URSA group compared to the control group.

Fig. 2. The independent sample t-test was used to compare the mean percentage of HLA class I and II in the URSA and control groups. *: Considered significant in comparison with the control group. The mean percentage ± SD of HLA class I in the URSA group is 10.1±2.2, and in the control group is 29.9±1.6; P-value is 0.0001. The mean percentage of HLA class II in the URSA group is 14.3±2.5, and in the control group is 34.4±1.8; P-value is 0.0001.
The alteration of spermatozoa antigenicity can lead to pregnancy complications, such as URSA (18). Accordingly, this study showed that there is a decreased expression of HLA class Ⅰ and Ⅱ on spermatozoa in RSA couples. To the best of our knowledge, no researcher has addressed the HLA class Ⅰ and Ⅱ expression on spermatozoa in couples with RSA.
There are old and very conflicting data in the field of polymorphic HLA expression by spermatozoa, and it has not yet been established whether polymorphic HLA expresses by spermatozoa. We think that using an insensitive and inappropriate technique for the detection of molecules with low expression can be the reason of this contradictory result. Among studies in this field, only a few of them evaluated the association between polymorphic HLA expression by spermatozoa and infertility (16, 17), and as far as we know, only one of them could show a correlation between HLA class Ⅱ on spermatozoa and infertility (17). However, in the mentioned study, all samples (in both fertile and infertile groups) did not express HLA class Ⅱ. The number of men whose spermatozoa expressed HLA class Ⅱ was higher in the infertile group than in the fertile group. Therefore, it suggests that there is a positive correlation between HLA class Ⅱ and infertility.
As we stated above, no study has been shown the relation between polymorphic HLA antigen and URSA. The present study is the first investigation that aimed to determine this relationship. This finding can be considered as a newly discovered cause of URSA. However, further investigations are necessary for determining the cause of alteration of HLA expression on spermatozoa and also determining why and how this alteration can eventuate RSA. The results of these investigations will support the discovery of a new therapy for URSA couples.
Regarding the induction of immunity by HLA antigens, most studies, especially in the field of transplantation, have shown that allogeneic HLA antigens induce the effector mechanism rather than the regulatory mechanism of the immune response. Also, this condition occurs in URSA women. Therefore, we hypothesized probably one of the reasons of enhanced effector immune responses in URSA couples may be a result of the increased expression of HLA antigen on spermatozoa. However, our study was opposite to our hypothesis. The evidence suggests that semen and spermatozoa must be able to stimulate immunity in the FRT strongly. Therefore, the expression of HLA antigens by spermatozoa, as a strong stimulator of immunity, may be essential for strong stimulation of immunity in the FRT. The fate of this immune response is a regulatory dominant immune response. We think that the decreased expression of HLA by spermatozoa can lead to disturbing this process and unsuccessful pregnancy.
In addition to the immune-stimulatory role of the HLA expression by spermatozoa, there is a possibility that HLA on spermatozoa has some roles in the fertility processes, such as sperm capacitation, acrosome reaction, and fusion with the oocyte. Nonetheless, further studies should be performed for determining the role of HLA antigens, their variants (presented by spermatozoa in the reproduction and pregnancy complication), and mechanism of action.
The limitation of our research is that a small sample size reduces the power of the study. Despite this limitation, we believe that our work could be a starting point for future investigations and introduction of new causes for URSA.
Our work has led us to conclude that the decreased expression of polymorphic HLA class Ⅰ and Ⅱ by spermatozoa can be related to occurring URSA. Although this study revealed only a relation between URSA and HLA expression by spermatozoa, further studies are necessary for determining the role and action mechanism of HLA antigens on spermatozoa in fertilization processes. However, we believe that our study is the first step toward discovering new causes for URSA and new therapy for this pregnancy complication.
The authors would like to thanks Dr. Hossein Motedayen and Mohadeseh Thogyani and Mohammad Sadegh Hesamian for their valuable assistance in guiding subjects for giving samples. This work was financially supported by Isfahan University of Medical Sciences. The number of the ethics committee approval letter is 395480.
The authors declared that there is no conflict of interest regarding the publication of this article.
| Article View | 1,304 |
| PDF Download | 807 |