Document Type : Case Reports
Authors
Dept. of Pathology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Keywords
Hemangiopericytoma (HPC) was first described by Stout and Murray in 1942 (1). In the 2007 WHO Classification of Tumors of the Central Nervous System, meningeal hemangipericytoma is a rare neoplasm and constitutes about 0.4% of CNS tumors (2). Initially, its histological resemblance of meningioma caused HPC to be classified as a subtype of this tumor entity (‘angioblastic meningioma’). But now, HPC was defined as an own tumor entity, because of distinctive clinical and histopathological features (3,4).HPC is more commonly located supratentorially and tends to occur in a younger age group, with average age at presentation of 38–42 years(5,6). Approximately 10% of HPCs occur in children and are slightly more common in males (1.4:1) (6, 7). Patient presentation depends on the location of the lesion, but symptoms commonly include headache, seizure, visual dysfunction, and motor weakness. HPCs are significantly less common than meningioma and their clinical behavior is more aggressive than that of benign meningiomcas. Moreover, they have a strong tendency for local recurrence and extracranial metastasis. Despite their distinctive clinical and histopathological features, sometimes it is difficult to differentiate (7, 8). Given the clinical, pathological and imaging similarities between Hemangiopericytoma and angioblastic/anaplastic meningioma and the necessity of differentiating these two (choosing the proper treatment and prognosis), we present a report of meningeal Hemangiopericytoma tumor in a 33-year-old female.
Case report
A 33-year-old female was admitted to hospital for a sudden seizure for the first time. She has no previous history of seizure or family special disease.
She had a 3 year history of weakness and numbness of left lower limb. The patient had no history of trauma or fever. The neurological examination revealed that power was decreased in the right lower limb when compared to the left lower limb. Atrophy was found in the muscles of her limbs.
Cranial computed tomography revealed a dural based sphenoid wing mass lesion measuring 5x3x0.5 cm. After the surgical removal of the mass, the specimen was sent to our pathology laboratory for histopathological examination. We performed simultaneously routine hematoxylin and eosin (H&E) staining. Histological examination revealed a neoplasm composed of proliferation of a staghorn vascular pattern of spindly cells with oval nuclei, inconspicuous nucleoli and moderate amount of eosinophilic cytoplasm arranged in fascicles with areas of hyalinizedstroma, myxoid changes and type of vascular spaces. No necrosis is identified in our prepared sections. Mitotic activity is also noted (About 4-5 per 10 high power fields). All resected tumors had well developed reticulin fibers. Based on histomorphological examination (figure 1, 2), we needed an IHC study to confirm the diagnosis. So the panel of immunohistochemical studies including Progestrone receptor (PR), epithelial membrane antigen (EMA), and CD 34, Ki-67 antigen and Bcl-2 was performed.
Immunohistochemical stains were performed with the Dako kits (Dako North America, Inc., CA, USA), and the following antibodies were used: CD34 (QBEnd10; Immunotech, Marseille, France), epithelial membrane antigen (E29; Dako), B cell lymphoma 2 (bcl 2) (Dako), Ki 67 (MIB 1, DakoCytomation).
The IHC study revealed positivity with Ki-67 in 5% tumor cells, indicating proliferative activity ,bcl 2 (focal)and CD99, and negative results for PR and EMA.CD 34 was positive in few tumoral cells (figure 3, 4). Light microscopic findings and IHC study confirm the diagnosis of meningeal hemangiopericytoma, WHO grade II to III.
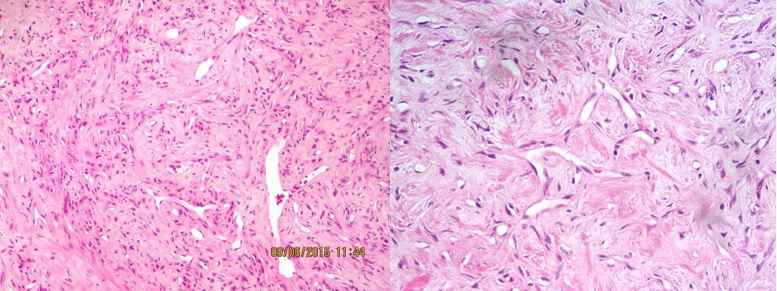
Fig 1, 2: diffuse proliferation of polygonal and spindled tumor cells with round or ovoid nuclei between blood vessels that showing the `staghorn'' shape. H&E stain, (×200)
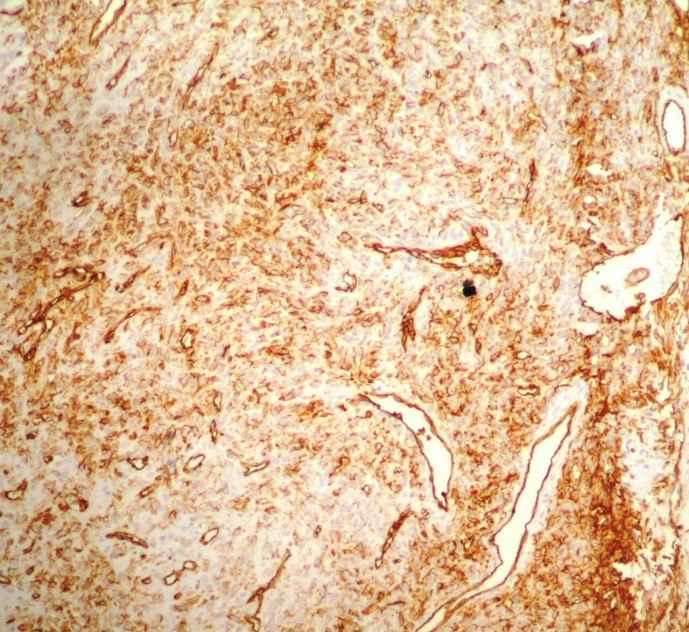
Fig 3: IHC study revealed positivity tumor cells with CD 34 marker. (×200)
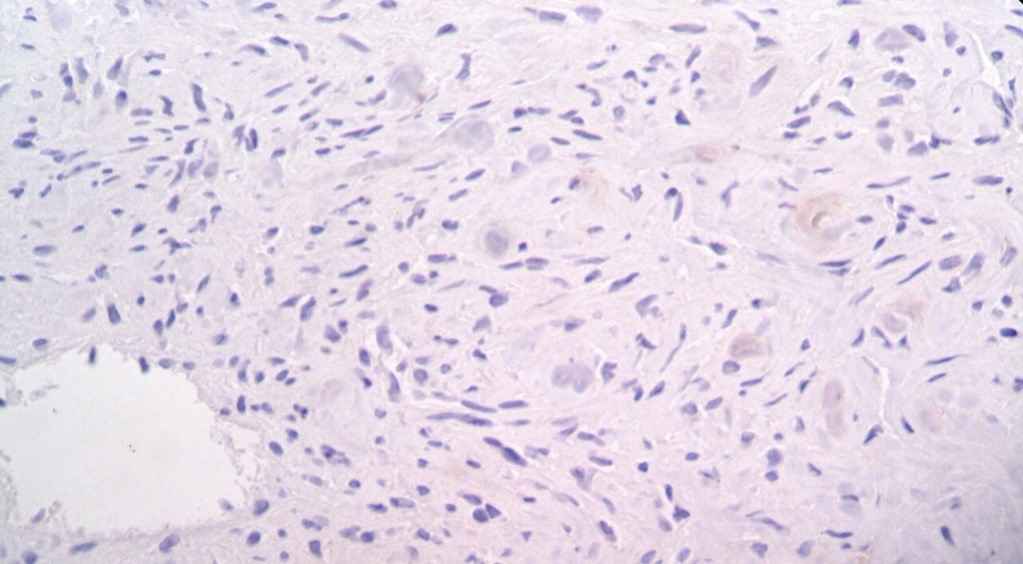
Fig 4: IHC study revealed negative tumor cells with EMA marker. (×200)
Discussion:
Meningeal hemangipericytoma is a rare neoplasm and constitutes about 0.4% of CNS tumors (8).
In previous studies on intracranial HPCs, the mean age at the time of diagnosis ranged from 38 to 50 years, which was lower than that of meningiomas (7).In our case report, the age was 33 years.
Headache is the commonest symptom in meningeal HPCs. A slight male preponderance is reported, with most cases occurring in the middle-aged group (7-9).On imaging, most intracranial HPCs are supratentorial in distribution; the commonest location is the parasaggital area. Almost all tumors have lobulated margins and are dense on CT. HPCs appear marked contrast enhancement on CT and MRI (10, 11). The main differential diagnosis of HPC includes angiomatous/anaplastic meningiomas and Solitary Fibrous Tumor. Meningeal hemangiopericytomas are significantly less common than meningioma. Many previous studies have discussed the differential diagnosis between meningeal hemangiopericytomas and meningiomas (8-10). Some studies reported that intracranial meningeal hemangiopericytomas are more multi-lobulated than benign meningiomas, and have thin based dural attachment on CT-scan and MR images (11, 12). Distinguishing HPCs from benign meningiomas before surgery also can be difficult, but is very important because of the aggressiveness of HPCs and their high rates of local recurrence and distant metastasis (13).
HPC’s clinical behaviors are more aggressive than that of benign meningiomas and have a strong tendency for local recurrence and extracranial metastasis. So differentiating these two entitles is vital. Despite their distinctive clinical and histopathological features, sometimes it is difficult to differentiate.
Histopathologically HPC shows a staghorn vascular pattern of spindly cells (14). At times, the histopathologic features of a HPC and meningioma can overlap. IHC can be helpful in this situation. Immunohistochemistry staining for MHPC show an intense reactivity to vimentin but not to epithelial membrane antigen (EMA), unlike meningioma that is positive for vimentin and EMA. CD34 appear focal or weakly staining in the HPC (15, 16). Bcl-2 seems to help differentiate these two entities. CD99 seems to be a good marker for HPC with specificity about 84% specificity.MHPC also shows a negative reaction to S-100 protein, factor VIII, CD31 as well as progesterone receptor (PR) (15, 16).
Conclusion:
Differentiation between HPCs and meningiomas is necessary for formulating a treatment strategy. Our study suggests that in addition to routine histopathological examination, immunohistochemical study is essential to differentiate it from angiomatous/anaplastic meningiomas and solitary fibrous tumor.
Acknowledgment
The authors declare that there is no conflict of interests.
Funding
The authors declare that there is no funding.
Conflict of Interest
The authors declare that there is no Conflict of Interests.
| Article View | 1,815 |
| PDF Download | 1,417 |