Document Type : Case Reports
Authors
1 Dept. of Pathology, Meenakshi Medical College Hospital & Research Institute, Enathur, Near Kanchipuram, Tamil Nadu
2 Dept. of Pathology, Meenakshi Medical College Hospital & Research Institute, Enathur, Near Kanchipuram, Tamil NaduV
Abstract
Mucinous carcinoma of the breast is a well-differentiated type of adenocarcinoma accounting for 2-5% of all breast cancers. Pure mucinous carcinoma of the breast has a favorable prognosis, usually seen in post-menopausal women. Neuroendocrine differentiation has been described in both in-situ and infiltrating breast cancers .Mucinous carcinomas of the breast appear to have the greatest association with neuroendocrine differentiation. Chromogranin A and synaptophysin are specific immunohistochemical markers of neuroendocrine differentiation. We report a case of mucinous carcinoma of the breast with neuroendocrine differentiation in a 67-year-old female who was treated surgically in a classical manner. How to cite this article: Varadharajan E, Priya S, Prakash G, Mugundan A, Easwaramurthi P. Mucinous Carcinoma of the Breast with Neuroendocrine Differentiation. Iran J Pathol. 2015;10(3):231-6.
Keywords
Introduction
Among the all-primary carcinomas of the breast, the incidence of mucinous (colloid or gelatinous) carcinomas of breast accounts for 1.5 to 5% (1). It is a well-recognized type of infiltrating ductal carcinoma, occurring mainly in elderly women and carrying a good prognosis. In general mucinous carcinomas are defined as having >50% of mucin production (2)
The following is a case report of a 67 year old female having mucinous carcinoma with neuroendocrine differentiation subsequently confirmed by immunohistochemistry (IHC). The case was presented to emphasize that whenever neuroendocrine component is noted it is important to differentiate between breast carcinomas with neuroendocrine differentiation and pure neuroendocrine tumours of the breast (which may be primary or metastatic) and extensive sampling and immunohistochemistry for neuroendocrine markers is necessary to differentiate between these two conditions.
Case Report
A 67 yr old post-menopausal woman presented to MeenakshiMedical College Hospital, Kanchipuram, Tamilnadu with complaints of swelling in the left breast for 1 year. The tumor gradually increased in size, which originated in the upper outer quadrant of the left breast measuring 5×4cm and was soft and firm in consistency. Axillary nodes were enlarged and largest measured was 1 cm in diameter. Computed tomography of chest, ultra sonogram abdomen and bone scan did not show any evidence of secondary deposits. Cytological examinations were positive for malignancy. The patient underwent Modified Radical Mastectomy (MRM) and specimens were sent for histopathological examinations.
Specimen of modified radical mastectomy was received and measured 16×10×3cm with skin ellipse measuring 13×8cm. Cut surface showed a tumor measuring 7×6×5 cm solid gelatinous mass (Fig. 1). Total 5 lymph nodes were dissected with the size ranging from-0.5cm-1cm. Histopathological examinations showed tumor cells being arranged in a lobular pattern with clusters of tumor cells floating in pools of extracellular mucin (Fig. 2). The tumor cells were mildly pleomorphic with moderate amount of eosinophilic cytoplasm with vesicular nuclei, salt and pepper type of chromatin arranged in sinusoidal and insular pattern (Fig. 3). All the surgical margins were free. Out of the five lymph nodes dissected, two were positive for secondary metastatic deposits with the picture resembling primary breast cancer. The histopathological picture was suggestive of hyper cellular variant of mucinous carcinoma with neuroendocrine differentiation. Immunohistochemistry was performed on representative blocks and was found positive for neuron specific enolase(NSE) Chromogranin A (CGA) and synaptophysin(SYN) (Fig. 4). Tumor was positive for estrogen receptor (ER) and progesterone receptor (PR) and negative for human epidermal growth factor receptor (HER2/neu) (Fig. 5). Taking under consideration of all above-mentioned findings a final diagnosis of mucinous carcinoma of the breast with neuroendocrine differentiation was given.
There is no conflict of interest in regards of ethical issues as informed consent was taken by the department of surgery.

Fig. 1: Gross photograph of mastectomy specimen showing gelatinous mass (Original)

Fig. 2: Photomicrograph showing clusters of tumor cells floating in extra cellular pools of mucin. (H&E, ×100)

Fig. 3: Photomicrograph showing tumor cells arranged in sinusoidal pattern (H& E, ×100)
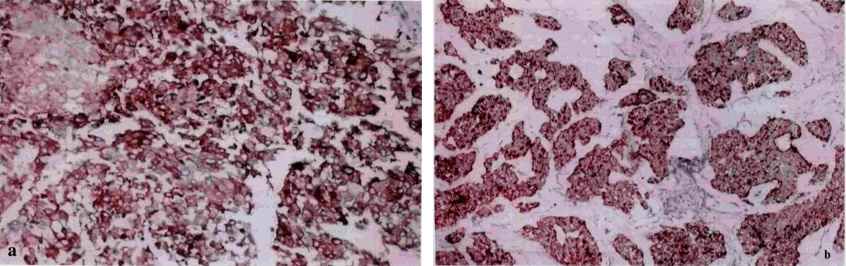

Fig.4: Photomicrograph of mucinous carcinoma of the breast showing strong positivity for synaptophysin ;A)chromogranin A ;B) neuron specific enolase ; C) in the areas of neuroendocrinedifferentiation. (IHC ×100)

Fig.5: Photomicrograph of mucinous carcinoma showing HER2/ neu negative (IHC ×400)
Discussion
Neuroendocrine tumors are common in the gastrointestinal tract and in the respiratory system. The incidence of neuroendocrine differentiation in breast carcinomas is 2-5% and pure neuroendocrine carcinoma is even rarer with an incidence of less than 0.1% of all breast tumors (3-5).
Literature search shows the presence of neuroendocrine cells lying among epithelial and myoepithelial cells .Neuroendocrine cells produce specific products like Chromogranin A and synaptophysin (3). Breast carcinomas, which show neuroendocrine differentiation, are mucinous carcinoma, ductal carcinoma, infiltrating lobular carcinoma, low-grade insular carcinoma, small cell undifferentiated carcinoma and ductal carcinoma in situ of which mucinous carcinoma has the greatest association with neuroendocrine differentiation (6).
Gary et al. (6) have described 38 cases of neuroendocrine differentiation in mucinous carcinoma, with an age ranging from 35-101 years (mean age-66yr). Our case has the similar age group.
Kashiwagi et al. (7) have reported 71 cases of mucinous carcinoma. In their study, the tumor size ranged from 0.5cm-12.3cm with mean tumor diameter-3.1cm. In the present case, the size of the tumor was 7cm.
Grossly mucinous carcinomas were well circumscribed with soft gelatinous consistency. Histologically it is classified into two subgroups – pure type and mixed type. The pure type is the classical type composed entirely of mucinous carcinoma and can be further subdivided into cellular and hypocellular variants based on the degree of cellularity. Hypocellular variant shows a tubular, cribriform, cord-like, micropapillary or papillary growth pattern, and hypercellular variant grows in solid nests. The tumor cells in pure mucinous carcinoma show mild pleomorphism. When the mucinous component is mixed with another tumor type mostly with ductal type, it is referred as mixed type of mucinous carcinoma (7). Our case shared features of cellular variant of mucinous carcinoma without any other ductal component.
The cellular variant of mucinous carcinoma frequently shows neuroendocrine differentiation. The morphological features of neuroendocrine differentiation are the arrangement of the tumor cells in solid sheets and in insular pattern, stippled chromatin, low-grade cytological features and positive neuroendocrine markers in less than 50% of the tumor cells. Chromogranin A and synaptophysin are widely accepted specific immunohistochemical markers of neuroendocrine differentiation.(3-5) Our case had similar histological features and immunohistochemistry positive for Chromogranin A, synaptophysin and neuron specific enolase in 30% of the tumor cells.
Lymph node metastasis is rare in mucinous carcinomas. Axillary nodal metastasis has been reported in both pauci cellular and hyper cellular variant of pure mucinous carcinoma (6,7). 3-15% of the pure mucinous carcinomas may show axillary nodal metastasis compared to 33-46% of the mixed type (8).The present case also has axillary nodal metastasis.
Kashiwagi et al.(7) have done breast cancer sub typing in 71 cases of mucinous carcinoma based on the HR and HER2 expression and showed that, of the 71 tumors, 68 (95.8%), one (1.4%) and two (2.8%) were HR-positive/HER2-negative, HR-negative/HER2-postive and HR-negative/HER2-negativerespectively. In our case, the hormone receptor status of the tumor showed ER+/PR+ and HER2/neu negative. ER+/PR+ tumors have a better prognosis than tumors with high levels of HER2/neu. HER2/neu is a negative prognostic factor expressed in 20-30% of breast cancers (9). In general, mucinous carcinoma with neuroendocrine differentiation shows good prognosis however, tumors with over expression of HER2/neu have increased recurrence and metastasis. Adair et al. (9) have reported a case of mucinous carcinoma with mediastinal vessel invasion, aggressiveness being attributed due to over expression of HER2/neu.
The present case has to be differentiated from pure neuroendocrine carcinoma of the breast. In 2012, WHO divided carcinomas with neuroendocrine features into three categories: neuroendocrine tumor, welldifferentiated; neuroendocrine carcinoma, poorly differentiated/small cell carcinoma; and invasive breast carcinoma with neuroendocrine features. The presence of in situ component in the breast, exclusion of other primary sites and positivity for neuroendocrine markers in more than 50% of the tumour cells will differentiate primary neuroendocrine carcinoma from breast carcinoma with neuroendocrine features (10).
Though Neuroendocrine differentiation has long been described its significance is still unknown. Gary Good prognosis was found in mucinous carcinoma of the breast showing neuroendocrine differentiation (6). It is difficult to make a prognosis for primary neuroendocrine carcinoma of the breast due to lack of long-term survival data. Primary neuroendocrine carcinoma of the breast carries a worse prognosis (10,11).
Although the breast is a rare location for primary neuroendocrine tumors, but it may be seen in the form of neuroendocrine differentiated carcinoma as seen in present case with mucinous carcinoma. Though mucinous carcinoma with neuroendocrine differentiation carries a good prognosis, as for any breast carcinomas the size of the tumor and hormone receptor sub typing are important prognostic factors in the present case also. Immunohistochemistry with neuroendocrine markers is essential when there is insular or nested pattern of growth to differentiate from primary neuroendocrine carcinoma of the breast, which carries a poor prognosis.
Acknowledgement
We acknowledge Dr. Lal Path, National Reference Labs,New Delhi for conducting the immuno histochemical tests synaptophysin and chromogranin A. The authors declare that there is no conflict of interests.
How to cite this article:
Varadharajan E, Priya S, Prakash G, Mugundan A, Easwaramurthi P. Mucinous Carcinoma of the Breast with Neuroendocrine Differentiation. Iran J Pathol. 2015;10(3):231-6.
- Sangeeta B Kulkarni ,Kanchanmala G Ghorpade,Ila M Vora, Srivastava S. Metastasis of Mixed Mucinous Carcinoma of Breast to Axillary Lymph Node. Bombay Hosp J 2006;48(1):183-6.
- Ramraje S, Ansari S, Sisodia S, Chaturvedi N, Bhatia V,Goel A. Pure Mucinous Carcinoma of the Breast. Iran J Pathol 2013;8(3):199 – 203.
- Gündüz M, Iscan Y, Erbil Y, Müslümanoglu M.Neuroendocrine differentiated breast carcinoma: a case report. J Breast Health 2009;5(4):225-7.
- Akhtar K, Zaheer S, Ahmad S S, Hassan M J. Primary neuroendocrine carcinoma of the breast.Indian J. Pathol. Microbiol 2009;52(1):71-3.
- Saeed A, Rehman A,Amjad H Zaidi, Shaukat T, Jamil K, Abdullah K.Neuroendocrine Carcinoma of Breast. J Coll Physicians Surg Pak 2011; 21(6):371-3.
- Gary MK Tse, Tony KF Ma, Winnie CW Chu, Wynnie WM Lam, Cycles SP Poon, Wing-Cheong Chan. Neuroendocrine differentiation in pure type mammary mucinous carcinoma is associated with favorable histologic and immunohistochemical parameters. Mod Pathol 2004; 17(5):568-72.
- Kashiwagi S, Onoda N, AsanoY, Noda S, Kawajiri H, Takashima T, et al.Clinical significance of the sub-classification of 71 cases mucinous breast carcinoma. Springerplus2013 23;2:481.
- Nilay C, Shalaka I, K H, Agarwal A. A rare case of mucinous carcinoma of thebreast. The Internet JSurg 2012; 28(4). http://www.ispub.com/journal/the-internet-journal-of-surgery/.
- Adair JD, Harvey KP, Mahmood A, Caralis J, Gordon W, Yanish G.Recurrent pure mucinous carcinoma of the breast with mediastinal great vessel invasion: Her-2/neu confers aggressiveness. Am Surg2008;74 (2): 113-6.
- Tajima S, Horiuchi H. Neuroendocrine tumor, well differentiated, of the breast: a relatively high-grade case in the histological subtype. Case Rep Pathol 2013; 2013: 204065.
- Nawawi O, Goh K Y, Rahmat K. A rare case of infiltrating primary neuroendocrine carcinoma of the breast. Iran J Radiol 2012;9(4): 212-6.

